Tesamorelin
$151.80
Tesamorelin
Islip
Pickup available, usually ready in 24 hours
3 Grant Ave.
Ste. B
Islip NY 11751
United States
+16316770030
Tesamorelin is a synthetic growth hormone-releasing hormone (GHRH) analog developed to stimulate endogenous growth hormone (GH) secretion. It is FDA-approved for reducing excess abdominal (visceral) fat in patients with HIV-associated lipodystrophy. By increasing GH and IGF-1 levels, Tesamorelin promotes lipolysis while preserving lean body mass. It is also being investigated for broader metabolic and fat-reduction benefits, including potential applications in non-HIV-related obesity and non-alcoholic fatty liver disease (NAFLD).
Applications
- FDA-approved treatment for HIV-associated lipodystrophy
- Reduction of visceral adipose tissue (VAT)
- Potential fat loss in non-HIV individuals (investigational)
- Preservation of lean body mass during fat reduction
- Improvement of lipid profiles
- Support in reducing liver fat and managing NAFLD
- Possible protection against age-related muscle loss
Mechanism of Action
GHRH Receptor Activation: Tesamorelin binds to GHRH receptors in the anterior pituitary, stimulating the release of endogenous growth hormone
Increased GH and IGF-1 Secretion: Elevated GH promotes lipolysis, while IGF-1 supports muscle growth and repair
Selective Fat Reduction: Increases fat oxidation, converting fat into energy, particularly abdominal fat
Key Research Studies
1. Visceral Fat Reduction in HIV Lipodystrophy
- Study focus: Tesamorelin vs placebo over 26 weeks
- Findings: 15–20% VAT reduction with no significant subcutaneous fat loss
- Reference: OUP Academic
2. Improved Lipid Profiles
- Study focus: Blood lipid markers
- Findings: Lowered triglycerides and improved cholesterol ratios
- Reference: OUP Academic
3. Liver Fat Reduction (NAFLD relevance)
- Study focus: Tesamorelin’s effect on hepatic fat in HIV patients
- Findings: Up to 37% relative reduction in liver fat over 12 months
- Reference: JAMA Network, The Lancet HIV
4. Sustained VAT Reduction
- Study focus: 52-week treatment extension
- Findings: Continued therapy maintained fat loss and improved body image/lipid profiles
- Reference: NATAP, PubMed
5. Neutral Glucose Metabolism Effects
- Study focus: Glucose tolerance over time
- Findings: No clinically significant changes in glucose or insulin markers
- Reference: OUP Academic, PubMed
Biological Effects and Benefits
Visceral Fat Loss
Significant reduction in abdominal VAT, particularly in HIV patients
Lean Muscle Preservation
Increases IGF-1, helping retain muscle during fat loss
Lipid Profile Improvement
Reduces triglycerides and improves cholesterol ratios
Liver Fat Reduction
May benefit NAFLD and hepatic inflammation
Sustained Effects
Continued treatment maintains body composition changes
Neutral on Glucose Tolerance
Continued treatment maintains body composition changes
Molecular Structure
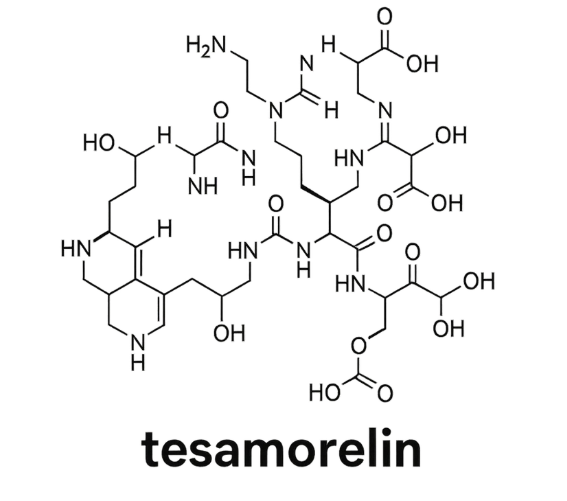
- Peptide Sequence: His-D-Ala-Asp-Ala-Ile-Phe-Thr-Gln-Ser-Tyr-Arg-Lys-Val-Leu-Gly-Gln-Leu-Ser-Ala-Arg-Lys-Leu-Leu-Gln-Asp-Ile-Met-Ser-Arg-Glu-Gln-Gly-Gln-Ser-Ala-Arg-Kys-Leu-Leu-Gln-Asp-Ile-Met-Ser-Arg-NH₂
- Molecular Formula: C₂₂₁H₃₆₆N₇₂O₆₇S
- Molecular Weight: 5135.85 g/mol
- Structure Notes: Long synthetic peptide mimicking endogenous GHRH, enabling stable subcutaneous administration and pituitary activation